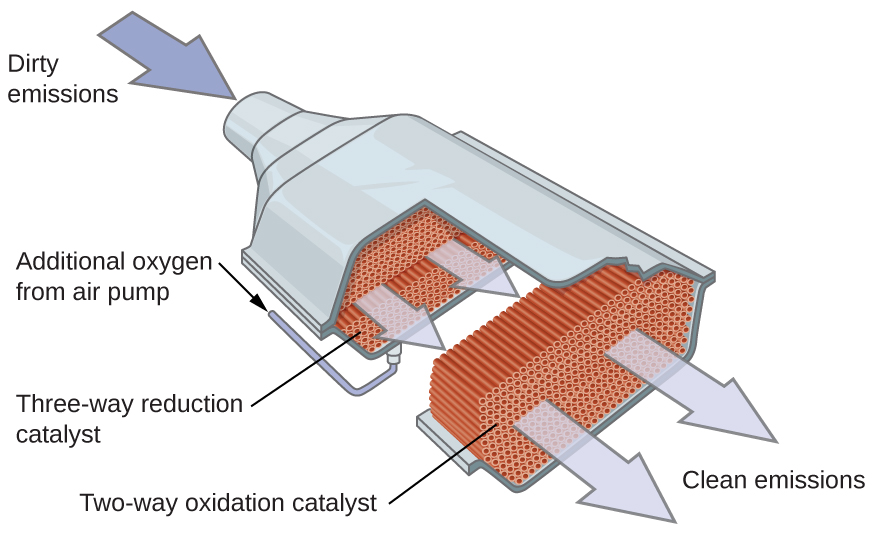
| << Chapter < Page | Chapter >> Page > |
Dr. Deanna D’Alessandro develops new metal-containing materials that demonstrate unique electronic, optical, and magnetic properties. Her research combines the fields of fundamental inorganic and physical chemistry with materials engineering. She is working on many different projects that rely on transition metals. For example, one type of compound she is developing captures carbon dioxide waste from power plants and catalytically converts it into useful products (see [link] ).
Another project involves the development of porous, sponge-like materials that are “photoactive.” The absorption of light causes the pores of the sponge to change size, allowing gas diffusion to be controlled. This has many potential useful applications, from powering cars with hydrogen fuel cells to making better electronics components. Although not a complex, self-darkening sunglasses are an example of a photoactive substance.
Watch this video to learn more about this research and listen to Dr. D’Alessandro (shown in [link] ) describe what it is like being a research chemist.

Many other coordination complexes are also brightly colored. The square planar copper(II) complex phthalocyanine blue (from [link] ) is one of many complexes used as pigments or dyes. This complex is used in blue ink, blue jeans, and certain blue paints.
The structure of heme ( [link] ), the iron-containing complex in hemoglobin, is very similar to that in chlorophyll. In hemoglobin, the red heme complex is bonded to a large protein molecule (globin) by the attachment of the protein to the heme ligand. Oxygen molecules are transported by hemoglobin in the blood by being bound to the iron center. When the hemoglobin loses its oxygen, the color changes to a bluish red. Hemoglobin will only transport oxygen if the iron is Fe 2+ ; oxidation of the iron to Fe 3+ prevents oxygen transport.
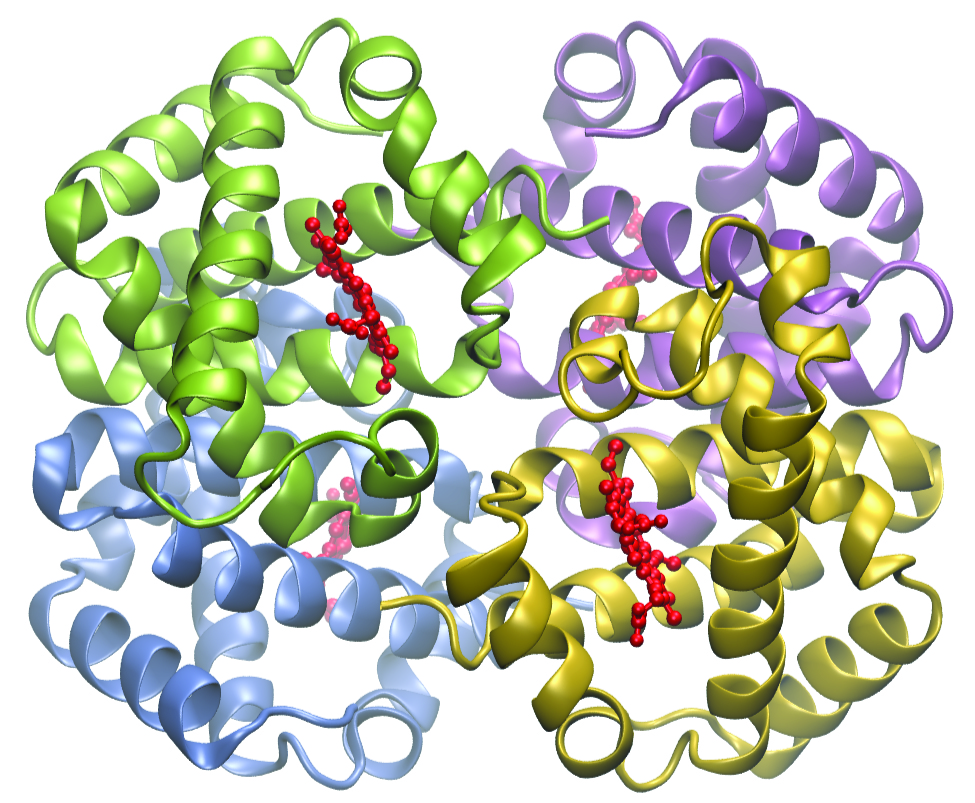
Complexing agents often are used for water softening because they tie up such ions as Ca 2+ , Mg 2+ , and Fe 2+ , which make water hard. Many metal ions are also undesirable in food products because these ions can catalyze reactions that change the color of food. Coordination complexes are useful as preservatives. For example, the ligand EDTA, (HO 2 CCH 2 ) 2 NCH 2 CH 2 N(CH 2 CO 2 H) 2 , coordinates to metal ions through six donor atoms and prevents the metals from reacting ( [link] ). This ligand also is used to sequester metal ions in paper production, textiles, and detergents, and has pharmaceutical uses.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?