| << Chapter < Page | Chapter >> Page > |
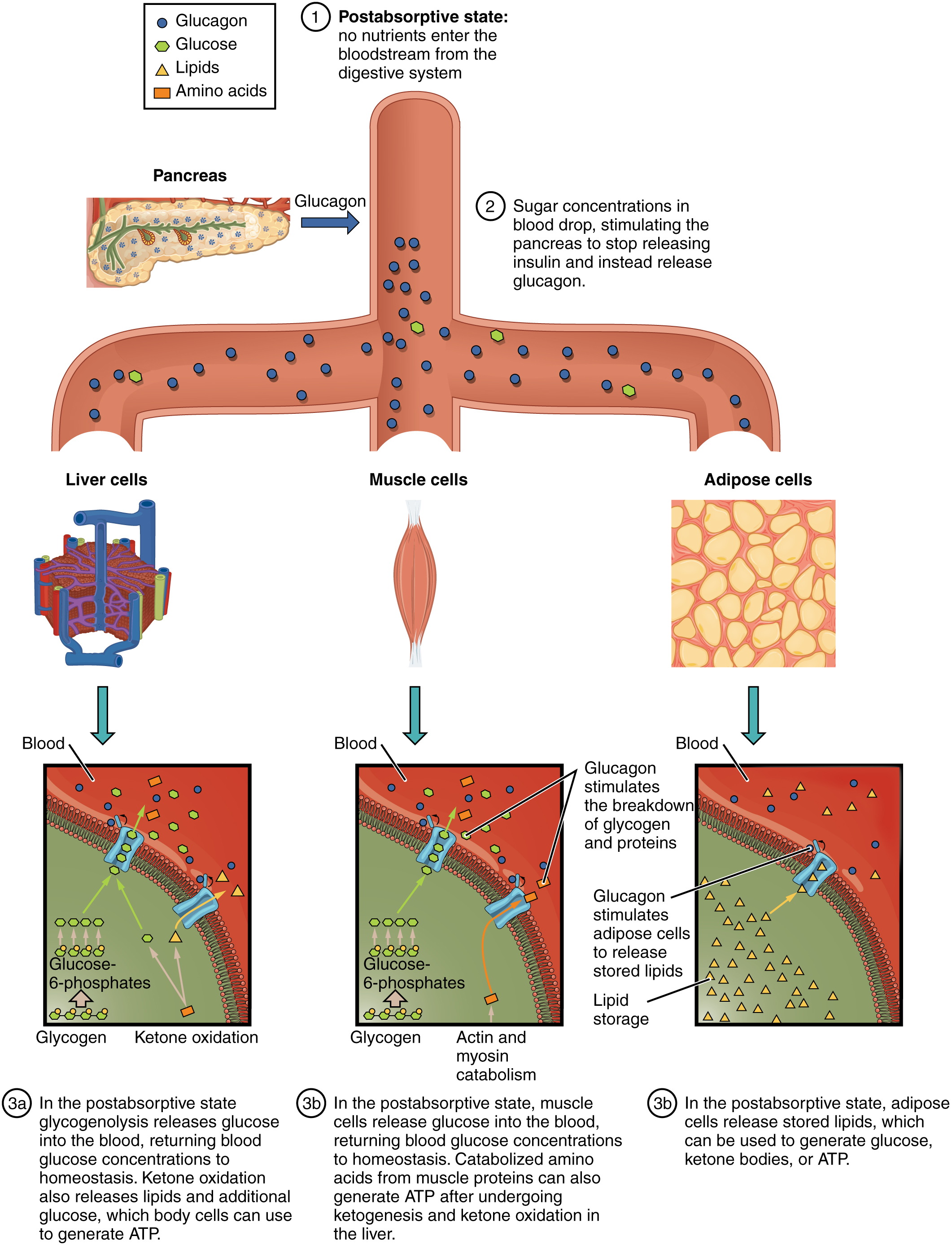
The postabsorptive state , or the fasting state, occurs when the food has been digested, absorbed, and stored. You commonly fast overnight, but skipping meals during the day puts your body in the postabsorptive state as well. During this state, the body must rely initially on stored glycogen . Glucose levels in the blood begin to drop as it is absorbed and used by the cells. In response to the decrease in glucose, insulin levels also drop. Glycogen and triglyceride storage slows. However, due to the demands of the tissues and organs, blood glucose levels must be maintained in the normal range of 80–120 mg/dL. In response to a drop in blood glucose concentration, the hormone glucagon is released from the alpha cells of the pancreas. Glucagon acts upon the liver cells, where it inhibits the synthesis of glycogen and stimulates the breakdown of stored glycogen back into glucose. This glucose is released from the liver to be used by the peripheral tissues and the brain. As a result, blood glucose levels begin to rise. Gluconeogenesis will also begin in the liver to replace the glucose that has been used by the peripheral tissues.
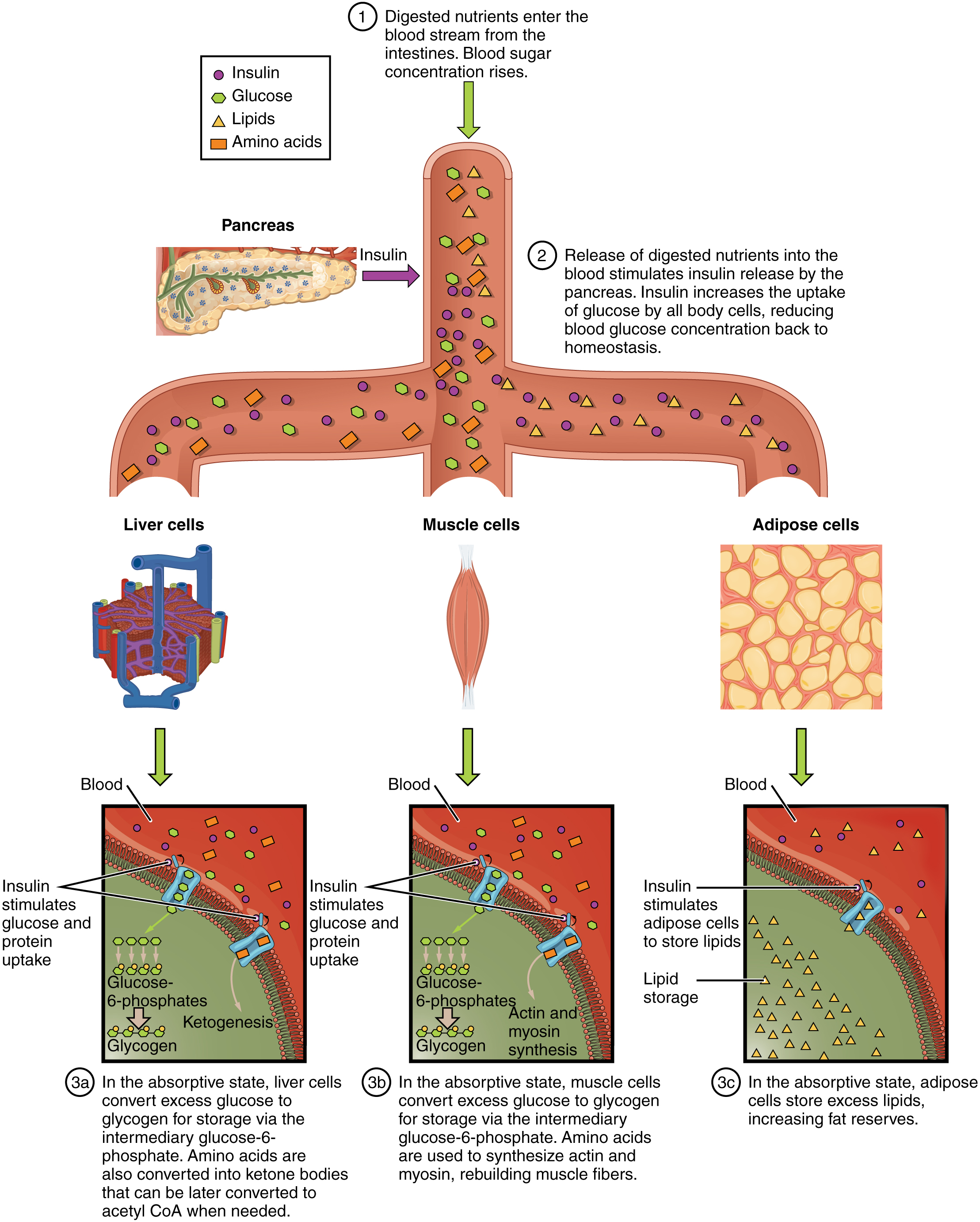
After ingestion of food, fats and proteins are processed as described previously; however, the glucose processing changes a bit. The peripheral tissues preferentially absorb glucose. The liver, which normally absorbs and processes glucose, will not do so after a prolonged fast. The gluconeogenesis that has been ongoing in the liver will continue after fasting to replace the glycogen stores that were depleted in the liver. After these stores have been replenished, excess glucose that is absorbed by the liver will be converted into triglycerides and fatty acids for long-term storage. [link] summarizes the metabolic processes occurring in the body during the postabsorptive state.
When the body is deprived of nourishment for an extended period of time, it goes into “survival mode.” The first priority for survival is to provide enough glucose or fuel for the brain. The second priority is the conservation of amino acids for proteins. Therefore, the body uses ketones to satisfy the energy needs of the brain and other glucose-dependent organs, and to maintain proteins in the cells (see [link] ). Because glucose levels are very low during starvation, glycolysis will shut off in cells that can use alternative fuels. For example, muscles will switch from using glucose to fatty acids as fuel. As previously explained, fatty acids can be converted into acetyl CoA and processed through the Krebs cycle to make ATP. Pyruvate, lactate, and alanine from muscle cells are not converted into acetyl CoA and used in the Krebs cycle, but are exported to the liver to be used in the synthesis of glucose. As starvation continues, and more glucose is needed, glycerol from fatty acids can be liberated and used as a source for gluconeogenesis.
After several days of starvation, ketone bodies become the major source of fuel for the heart and other organs. As starvation continues, fatty acids and triglyceride stores are used to create ketones for the body. This prevents the continued breakdown of proteins that serve as carbon sources for gluconeogenesis. Once these stores are fully depleted, proteins from muscles are released and broken down for glucose synthesis. Overall survival is dependent on the amount of fat and protein stored in the body.
There are three main metabolic states of the body: absorptive (fed), postabsorptive (fasting), and starvation. During any given day, your metabolism switches between absorptive and postabsorptive states. Starvation states happen very rarely in generally well-nourished individuals. When the body is fed, glucose, fats, and proteins are absorbed across the intestinal membrane and enter the bloodstream and lymphatic system to be used immediately for fuel. Any excess is stored for later fasting stages. As blood glucose levels rise, the pancreas releases insulin to stimulate the uptake of glucose by hepatocytes in the liver, muscle cells/fibers, and adipocytes (fat cells), and to promote its conversion to glycogen. As the postabsorptive state begins, glucose levels drop, and there is a corresponding drop in insulin levels. Falling glucose levels trigger the pancreas to release glucagon to turn off glycogen synthesis in the liver and stimulate its breakdown into glucose. The glucose is released into the bloodstream to serve as a fuel source for cells throughout the body. If glycogen stores are depleted during fasting, alternative sources, including fatty acids and proteins, can be metabolized and used as fuel. When the body once again enters the absorptive state after fasting, fats and proteins are digested and used to replenish fat and protein stores, whereas glucose is processed and used first to replenish the glycogen stores in the peripheral tissues, then in the liver. If the fast is not broken and starvation begins to set in, during the initial days, glucose produced from gluconeogenesis is still used by the brain and organs. After a few days, however, ketone bodies are created from fats and serve as the preferential fuel source for the heart and other organs, so that the brain can still use glucose. Once these stores are depleted, proteins will be catabolized first from the organs with fast turnover, such as the intestinal lining. Muscle will be spared to prevent the wasting of muscle tissue; however, these proteins will be used if alternative stores are not available.

Notification Switch
Would you like to follow the 'Anatomy & Physiology' conversation and receive update notifications?